- Industries & Machines Industries & Machines
- IIoT IIoT
- Service-Toll Processing Service-Toll Processing
- Material Material
- News News
- IR Information IR Information
-
Sustainability
Sustainability
Sustainability
- Introduction
- Hosokawa Micron Group "Basic Human Rights Policy"
- Sustaibality Policy - Mission Statement
- Editorial Policy
- Integrated Report
- Materiality & Strategy
- Technological contribution to a sustainable global environment
- Contributions towards a safer, more secure and prosperous society
- Sophistication of governance that supports business
- ESG Data Collection
- Sustainable Business Management ~ Finance
- Infromation Disclosure Based on TCFD Recommendations
- Jobs and Careers Jobs and Careers
-
About Us
About Us
About Us
- Greetings (Company Introduction)
- Hosokawa Micron Group "Basic Human Rights Policy"
- Management Philosophy
- Corporate Overview
- Corporate Profile
- Business Areas and Strengths
- Corporate History
- Hosokawa Micron Group
- Domestic Facilities
- Overseas Subsidiaries (Asia)
- Overseas Subsidiaries (Europe)
- Overseas Subsidiaries (America)
- Asian Agents
- Powder Technology Research Institute
- Industrial Property Rights
- Journals and Books
- Technical Information
- Annual Publication "Micromeritics"
- Compliance Charter
- Privacy Policy
- Cookie Policy
- Quality Principle

Industries & Machines
- TOP
- Industries & Machines
- Industries Search
- Isolator
Isolator

Summary
The active ingredients of pharmaceuticals are poisonous or dangerous when exposed to the human body in doses above proper quantities. For development and production of highly active pharmaceutical ingredients such as cancer drugs, special equipment is required.
Contents

Fig.1 Fine Impact Mill 250UPZ in pharam design
Quality Control and Operator Protection
For pharmaceutical production systems, not only it is necessary to have a quality assurance system such as GMP in place, but to also balance the occupational health and safety requirements of securing the safety of the operators. Operating the equipment without the risk of injury, prevention of dust explosions and in the case of highly active pharmaceutical ingredients countermeasures for chemical hazards is required. Protective clothing such as dust protective masks or isolator suits have been used as protection in the past, but with the development of pharmaceuticals with highly active ingredients and cases of drug induced diseases, guidelines from a risk management standpoint and occupational safety restrictions are being strengthened worldwide.
Isolator
An isolator is used to contain powder processing equipment, limit the area of contamination and preventing the exposure of dust with a high degree of reliability. On the other hand, operating equipment via gloves limits the layout and operability of equipment. Thanks to pioneering development work, Hosokawa Group companies have vast experience in this application. Hosokawa has delivered many isolators to various pharmaceutical companies not only meeting GMP requirements, but also with improved safety, reliability and ergonomically designed to be easy to use. Customization to meet customer requests is also taken into account in the design and fabrication process. The risk is especially high in the milling process of APIs where high concentrations of dried powder are processed.
A multitude of Isolator experience
Hosokawa has a multitude of experience supplying Multi-Systems that can handle various batch sizes, different milling systems (mechanical mill, jet mill) that can be used for initial development of new drugs, production of clinical trial drugs and mass production. The primary components are the intake HEPA filter, exhaust double HEPA filter, fan with automatic static pressure control, illumination, cleaning equipment, container capable of connection/detachment while keeping containment, and valves.Gloves are easy to manipulate and clean with a proprietary design glove port allowing easy and safe changes of gloves. Even if one glove is detached accidentally, the isolator is designed to prevent dust from leaking out of the isolator.
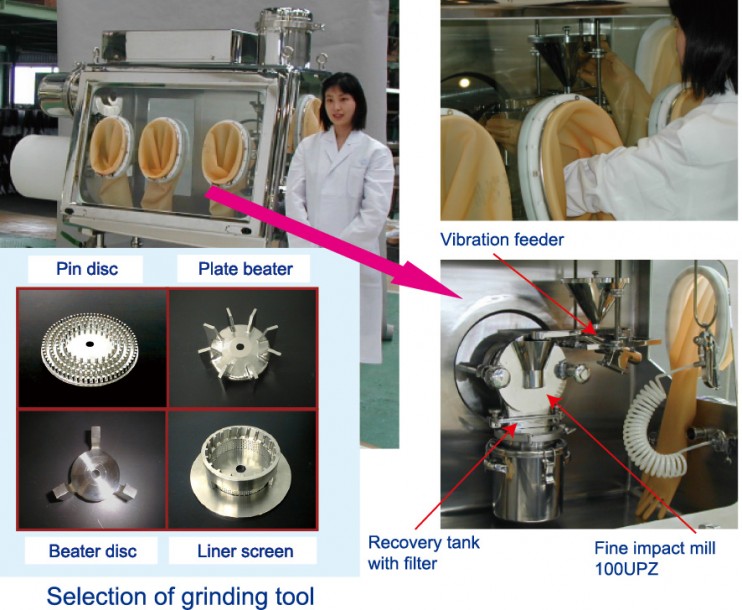
Fig.2 Isolator with Fine Impact Mill 100UPZ
Fine Impact Mill in Isolator
Fig.2 shows a Fine Impact Mill UPZ inside an isolator suitable for milling to below 100 μm.
Jet Mill in Isolator
Fig.3 and Fig.4 show jet mills suitable for ultra fine milling to several microns. The shaft seals are specially designed to meet the demands of GMP and safety. Operating the system according to designated procedures is required. The isolator system needs to be designed around the operational procedure of the required powder processing equipment.
Isolator Design and Fabrication
For safe and comfortable system operation, the design of the isolator and process equipment requires careful consideration. A drawing that fulfills all the requirement is drawn up and a wooden mock-up made. The ergonomics and workability is checked at this stage and if sufficient, the project moves to the final design stage.
By utilizing an isolator, the contaminated area can be limited to the inside of the isolator minimizing the contaminated area to a minimum.

Fig.3 Spiral jet mill in isolator

Fig.4 Fluidized bed opposed jet mill in isolator
Related equipments

Feel free to contact us. if you have any questions or concerns.










